RegSmart EuroDURG 2025
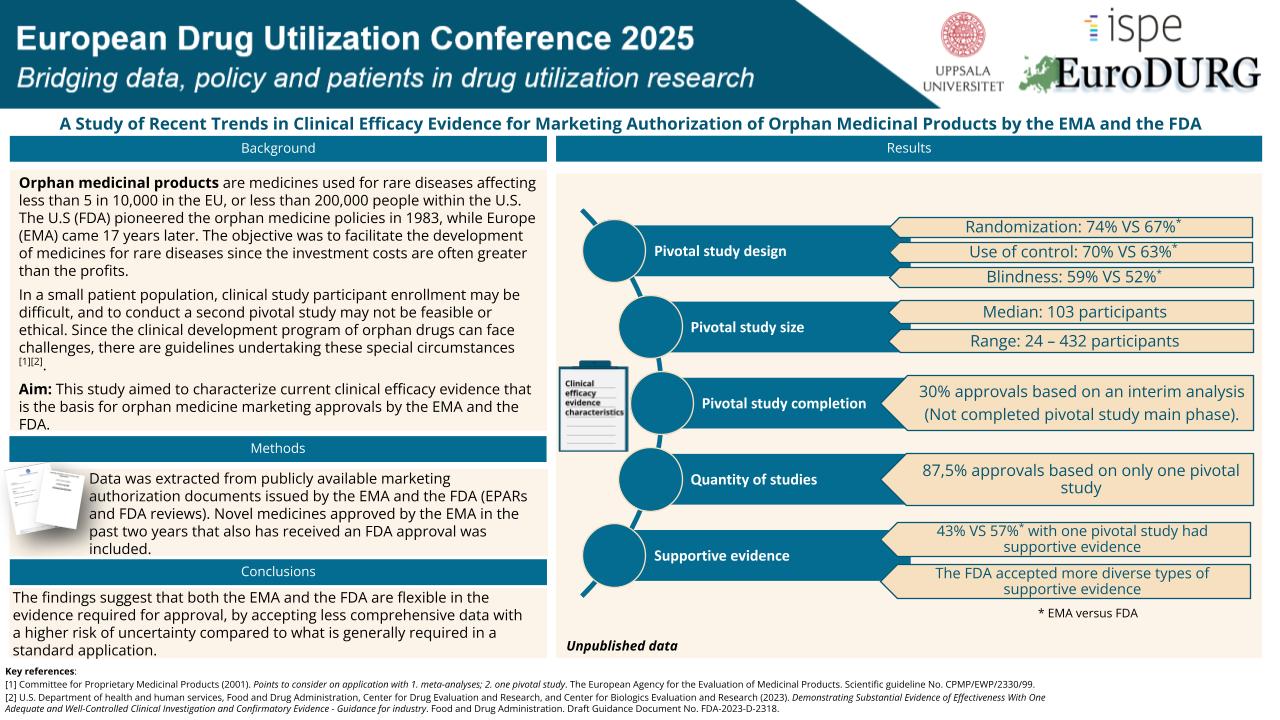
Early this summer, our former master’s thesis student Elise Nordenberg presented her project: “A Study of Recent Trends in Clinical Efficacy Evidence for Marketing Authorization of Orphan Medicinal Products by the EMA and the FDA”
At the EuroDURG 2025 conference in Uppsala. The project explored publicly available data from EMA and FDA, with focus on the clinical efficacy documentation in authorisations of orphans drugs, (treatments developed for rare diseases).
The findings show that while both agencies apply high standards, there is flexibility in the acceptance of efficacy data for small patient population.
This reflects a shared goal: ensuring timely access to new therapies for patients with high unmet needs.
Elise’s work is a great example of how regulatory science helps bridge innovation and access, and we’re very proud to have supported her in this important project.