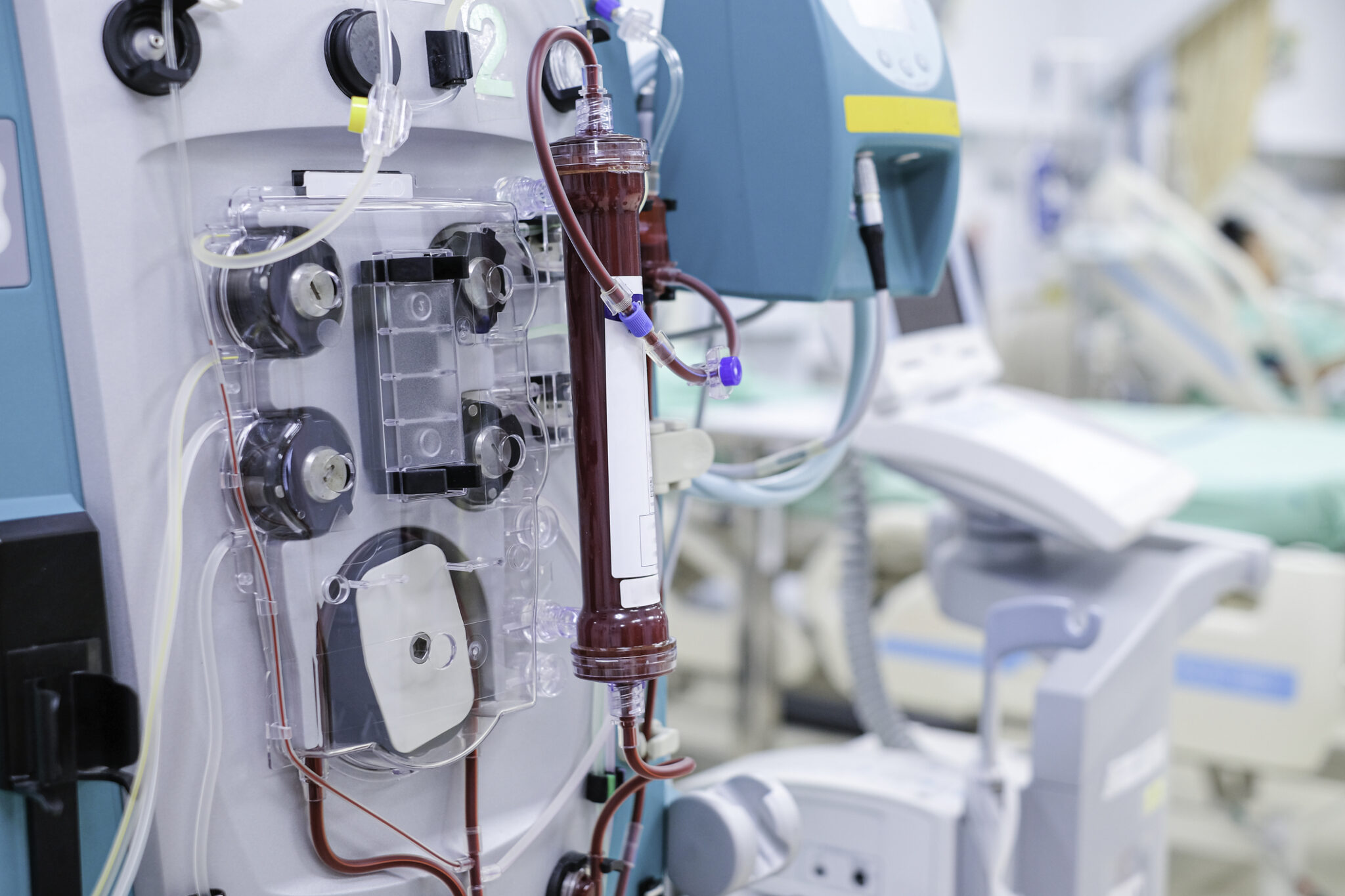
Triomed AB, a pioneering Swedish med-tech company, is developing the Carry Life UF system, bringing Steady Concentration Peritoneal Dialysis (SCPD) to patients – holding the promise of more effective, portable, home-based therapies. To support this groundbreaking initiative, Triomed has partnered with RegSmart Life Science AB, leveraging RegSmart’s rare blend of senior regulatory expertise in both pharma and MedTech.
Triomed AB is a Swedish MedTech company dedicated to revolutionizing peritoneal dialysis (PD) through its proprietary Steady Concentration Peritoneal Dialysis (SCPD) method. Their flagship platform, Carry Life UF, is designed to overcome limitations of conventional PD by enabling continuous ultrafiltration through a stable glucose levels, helping patients stay on PD longer, improving fluid and sodium removal and preserving membrane health. The Carry Life UF System is conducting pivotal clinical evaluation vital to receive regulatory clearances such as CE marking and Marketing Authorization (MA) from MPA. As Triomed advances toward regulatory milestones, RegSmart’s capability to navigate the overlapping regulatory frameworks of pharmaceuticals and medical devices has proven indispensable.
Marcello Grondelli, CEO of Triomed AB:
“RegSmart’s integrated expertise in both MedTech and pharmaceutical regulation has been instrumental in helping us define and navigate the regulatory pathway for Carry Life UF system. Their guidance enables us to stay agile in innovation while confidently advancing our clinical and regulatory development.”
Mats Högberg, Director MedTech/IVD at RegSmart Life Science AB:
“Triomed’s SCPD platform represents a meaningful step forward in dialysis therapy. Their innovative approach requires navigating a complex and challenging regulatory landscape, something we at RegSmart are both excited and proud to support. What sets us apart is our senior expertise across both pharmaceutical and medical device regulations – exactly what a boundary-breaking technology like Carry Life UF requires to move forward confidently.”